The Causes And Diagnosis Of Erdheim-Chester Disease
Erdheim–Chester disease is a rare cancer of the white blood cells. In this disease, excessive proliferation of macrophages causes damage to a variety of organ systems. Macrophages, the white blood cells that normally ingest foreign bacteria and viruses are overproduced and attack normal host tissues. The disease often attacks the long bones, the lungs, and the pituitary gland. It often presents with bone pain, but clinical presentation varies widely among patients.
The first cases were reported in 1930 by Jakob Erdheim and William Chester. As of 2017, there were 550 cases reported in literature. Symptoms range from mild to life-threatening depending on the organ systems involved. While no cure is known, some treatments can prolong survival and allow many patients to lead an almost normal life.Learn more about the causes and method of diagnoses of Erdheim-Chester disease.
Abnormal Inflammatory Process
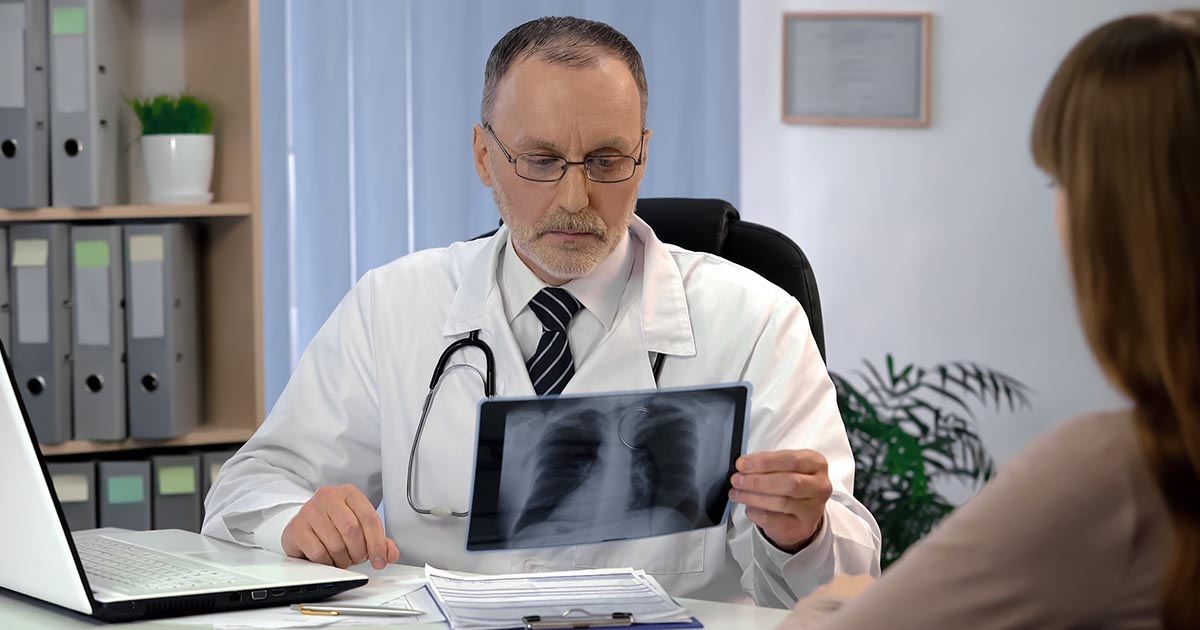
Erdheim-Chester disease is classified as an inflammatory myeloid neoplasm. The myeloid refers to white blood cells that arise from the bone marrow of the long bones, and neoplasm refers to the uncontrolled growth of the macrophages. Erdheim-Chester disease causes an abnormal inflammatory process that leads to scarring and fibrosis of connective tissue in multiple organ systems. Skeletal involvement is almost universal among patients, often presenting as symmetric pain in the long bones. Other organ systems involved include the lungs, brain, heart, and eyes. In patients with lung involvement, the inflammatory process leads to pulmonary fibrosis.
This reduced ability to oxygenate the blood causes labored breathing and can be life-threatening. When the macrophages infiltrate and damage the pituitary gland, diabetes insipidus can be the result. The pituitary gland helps regulate water balance by releasing the hormone vasopressin. Chronic inflammation causes excessive urination and the dilute urine typical of diabetes insipidus. In cases of cardiac muscle infiltration, the chemicals released by the inflammation can lead to cardiomyopathy. The heart becomes thicker with scar tissue and less able to pump effectively.
Gene Mutation

Erdheim-Chester disease is not an inheritable genetic condition, which means the mutation occurs at some point in an individual's life in a sub-population of the progenitor macrophage cells. Over half of all patients tested positive for the BRAF V600E mutation. The BRAF gene produces a protein involved in the RAS/MAPK cell signaling pathway. This pathway is important in controlling cell growth, proliferation, differentiation, and normal cell death.
The gene mutation produces an abnormally active faulty protein. This altered protein interrupts cell cycle controls and allows unregulated overproduction of the macrophages. Other mutations involving the MAPK and PIK3 pathways of the cell have been uncovered in recent studies. MAPK (mitogen-activated protein kinase) and PIK3 (Phosphoinositide 3-kinase) pathways are both cell signaling pathways involved in essential functions such as growth and division. Mutation in these genes is common among a variety of cancers.
